Particulate matter (PM) in the atmosphere is a mixture of solid particles and liquid droplets, many of which are not visible to the naked eye. PM can be directly emitted or formed by chemical reactions in the atmosphere. PM is a concern because it can 1) cause health impacts to humans, plants and animals, 2) degrade visibility, causing accidents on roadways or impairing the view of scenic vistas, and 3) deposit out of the atmosphere causing a variety of impacts including influencing nutrient cycles. Farming, ranching, and forestry operations can all be sources of particulate matter. This chapter is designed to give a brief introduction to PM characteristics and the impacts of PM.
Particulate Matter Size and Shape
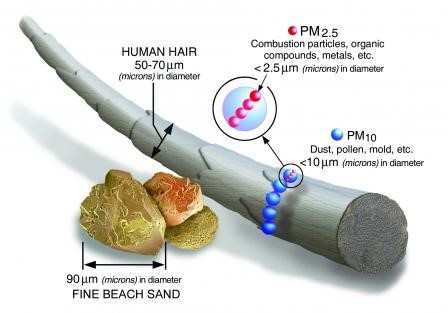
PM in the atmosphere exists in a wide range of shapes and sizes and is made up of a variety of chemical species (for example, carbon, sulfates, heavy metals). PM is classified by its size, where fine particles are the small particles that can be inhaled deep into the lungs. These fine particles are known as PM2.5, which are particles that have an aerodynamic equivalent diameter (AED) less than or equal to 2.5 micrometers (μm). The next size class of PM are inhalable larger particles, known as PM10, which are particles with an AED less than or equal to 10 μm. Note that PM2.5 is actually a subset of PM10. Particles are typically not perfectly spherical and instead come in a variety of shapes. The AED is defined as the diameter of a spherical particle with a density of 1 g/cm3 that would have the same settling velocity as the particle in question.1 Particles with the same AED presumably perform alike when suspended in the air. Particles larger than 10 μm in AED can also be suspended and transported in the atmosphere, however these particles usually settle out rather quickly in comparison to smaller particles. Figure 1-1 shows the relative size of PM2.5 and PM10 compared to a human hair and beach sand. PM2.5 is approximately 30 times smaller than human hair while PM10 is approximately 7 times smaller. PM concentration is typically measured on a mass per volume basis (μg/m3) but it can also be discussed in terms of particle number. While small diameter particles can have a low mass concentration in the atmosphere, on a particle number basis there can be orders of magnitude more fine particles than larger, more coarse particles.
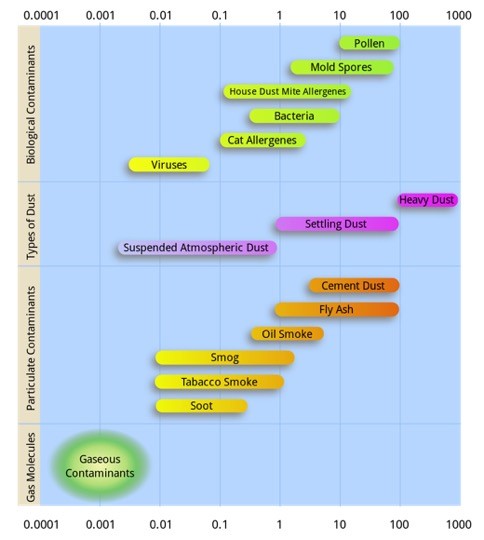
Fine PM concentrations in the atmosphere can be generated from a variety of natural and man-made sources, and can be comprised of carbonaceous compounds, soil (geologic material) and inorganic species of sulfate, nitrates and ammonium. Coarse PM typically is comprised of geologic dust or soil and often is directly emitted to the atmosphere. Fine PM can be directly emitted or created by physical and chemical processes in the atmosphere (secondary PM). Figure 1-2 shows examples of various types of particles and their typical size distributions. Dust tends to be 1-10 μm while combustion processes mostly produce smaller particles in the 0.01-1 μm range.
References
1. Baron Paul A, Willeke K. Aerosol Measurement: Principles, Techniques, and Applications. 2nd Ed. John Wiley & Sons, Inc.; 2001.
2. U.S. Environmental Protection Agency. Particulate matter (PM) basics. US EPA. https://www.epa.gov/pm-pollution/particulate-matter-pm-basics. Published April 19, 2016. Accessed July 11, 2019.
3. Montana State University. Nanoscience topics in earth science. Nanotechnology in STEM. https://serc.carleton.edu/msu_nanotech/nano_topics.html. Accessed July 11, 2019.
